
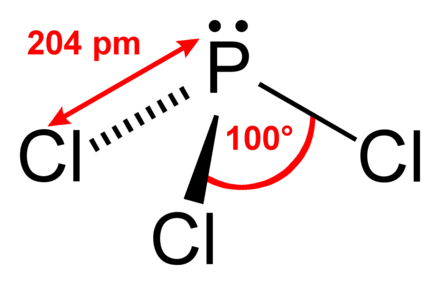
Hybridization is the process electrons in orbitals take up space, repel each other’s orbitals and alter the shape of a molecule. This is referred to as the dipole time (m). The polarity of molecules will be determined by the electronic shift towards one direction or another for neutral molecules. For example, based on the bond angle, the molecule could become linear, trigonal planar, tetrahedral, or octahedral. Phosphine is an important chemical with numerous applications and effects on the health of humans and the environment.īond angles of molecules are a crucial element in the structure of the molecules. Although it’s not a significant source of greenhouse gas emissions, it may harm wildlife and the environment when released in large amounts. Phosphine is extremely poisonous and should be handled with caution. It is created by the reaction of metal phosphide with acid and is utilized for reducing agents, fumigants, and a dopant in various industries. H3P, also known as Phosphine, is an extremely toxic gas with many commercial and industrial applications. In addition, the fumigation of phosphates specifically is known to have negative effects on species that are not targeted, including honeybees and beneficial insects. However, it could cause harm to wildlife and the environment if released in large amounts. Phosphine isn’t considered a major cause of greenhouse gas emissions because sunlight has a very short lifetime at the surface and rapidly destroys it. Therefore, Phosphine should be handled carefully and stored in a ventilated space away from fire-risk sources. In extreme cases, exposure to Phosphine may result in respiratory failure and even death. In addition, it can cause vomiting, nausea, and dizziness. It can irritate the nose, eyes as well as throat. Phosphine is a toxic gas that can lead to serious health issues when inhaled or inhaled via the skin. Phosphine also produces phosphorous-containing chemicals, such as flame retardants and pesticides.

For example, it is employed as a reduction agent in organic syntheses, as a fumigant in grain storage, and as a dopant in the manufacture of semiconductors. Phosphine has many commercial and industrial uses. The reaction produces an oxygen gas called Phosphine and an acid salt, usually, calcium chloride (CaCl2) or calcium sulfurat (CaSO4). Hydrochloric Acid (HCl) and sulfuric acid (H2SO4) are frequently used as acids. Calcium Phosphide (Ca3P2) is typically employed as the name of the metal phosphide. Phosphine is usually produced by reacting with a metal phosphide and an acid.


 0 kommentar(er)
0 kommentar(er)
